Photoelectric Effect, as the name suggests, is the emission of electrons from a material when light shines onto it. Such electrons are usually referred to as photo-electrons. This seemingly simple phenomena played a critical role in understanding the nature of light and the development of modern physics (quantum mechanics). In fact, Albert Einstein was awarded his Nobel prize in 1922 “for his services to theoretical physics, and especially for his discovery of the law of the photoelectric effect“, instead of for his theory of relativity.
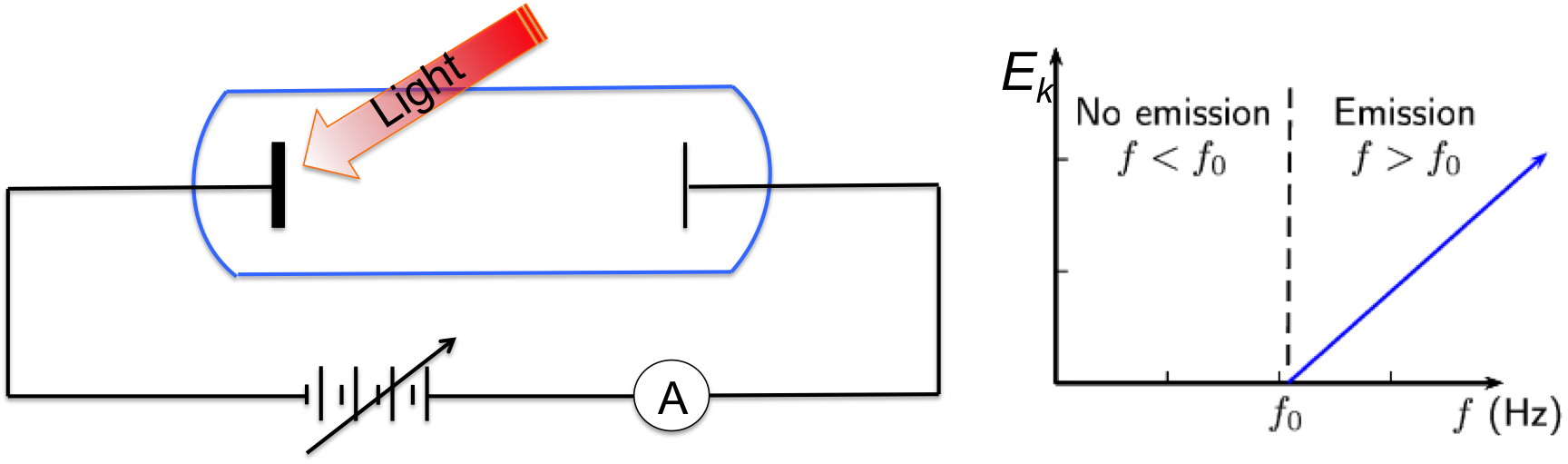
Figure 1, Setup for quantitative measurements of photoelectric effect, and the maximum kinetic energy of emitted electrons as a function of light frequency.
So, how does photoelectric effect occur, and how did it change our view about light? The setup used to evaluate photoelectric effect quantitatively is shown in Figure 1, where the material being investigated is sealed in a vacuum tube. A second plate is placed at the other end of the tube as the counter electrode. When light of certain frequency strikes the material, electrons may be emitted and they fly towards the counter electrode and produce current that can be measured by the amp-meter. To measure the kinetic energy of these emitted electrons, an adjustable DC voltage source is placed in the circuit. By adjusting the voltage till current become zero, one can obtain the kinetic energy of electrons being emitted from the material. This voltage is called the stopping potential (or cut off potential).
Facts: During the photoelectric measurement, couple of parameters can be varied, i.e. light frequency, light intensity, and DC voltage applied. The following facts can be observed:
1, For a given material, there exists a minimum light frequency (threshold frequency) below which no photoelectrons will be emitted, regardless of the light intensity.
2, Above the threshold frequency, the maximum kinetic energy of emitted electrons depends on the light frequency linearly, but is independent of the light intensity.
3, For a given material and incident light frequency, the rate at witch electrons are emitted is proportional to light intensity.
4, The time lag between the incidence of light and emission of electrons is very small, less than nano second.
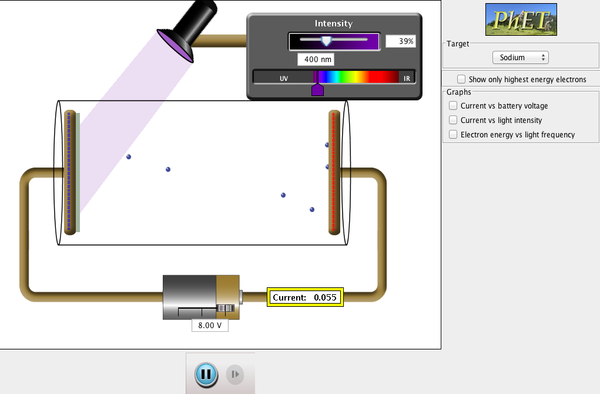
The following is a simulation that can help you to visualise these observations.