Cathode rays, as we now know, are streams of electrons generated by high electric field-induced gas ionization (old cold cathode ray tubes) or heat-induced thermionic emission (modern vacuum tubes) in specially prepared tubes. Such tubes are often referred to as cathode ray tubes.
Study of cathode rays began in the early 19th century, way before the identification of electrons. After the 1654 invention of the vacuum pump by Otto von Guericke, physicists began to experiment with passing high voltage through rarefied air. In 1705, it was already noted that electrostatic generator sparks travel a longer distance through low-pressure air than in atmospheric air.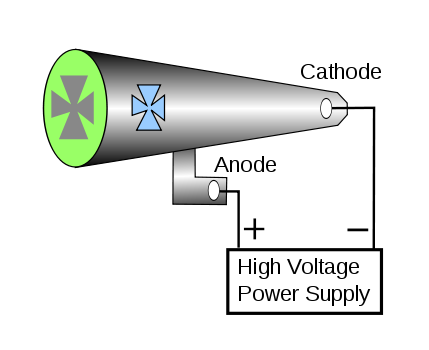
The early Geissler tubes. In 1838, Michael Faraday applied a high voltage through a glass tube filled with rarefied air and noticed a light arc starting from the cathode (the electrode connected to the negative end of the power supply) and ending at the anode (the positive electrode). In 1857, German physicist and glassblower Heinrich Geissler sucked more air out with an improved vacuum pump, to a pressure of around 10-3 atom. He observed, instead of an arc, a glow filled the whole tube. The voltage applied between the two electrodes was anywhere between a few kilovolts and 100 kilovolts and they were called Geissler tubes, similar to today’s neon signs. Of course, we know today that the light arc or glow in these tubes was not electrons, but produced by electrons when they travelled through the tube and struck the residual air atoms. After the strike, electrons within the atoms were excited, and when they returned to lower energy states, energy was released as electromagnetic waves (lights). Different elements have different characteristic atomic energy states, thus producing lights of different colors (neon lights).
The Crookers tubes. By the 1870s, British physicist William Crookes and others were able to evacuate the tubes to an even lower pressure, below 10-6 atom.

These were called Crookers tubes. Faraday was the first to notice a dark space just in front of the cathode, where there was no luminescence (known as “cathode dark space”, “Faraday dark space” or “Crookes dark space”). Crookes found that this darkspace expanded from the cathode towards the anode as he pumped more air out of the tube until the whole tube was dark. However, at the anode end of the tube, the glass wall itself began to glow. If an object were placed in the tube before the anode end, a shadow would be formed on the glass wall, suggesting some particles travelled from the cathode toward to anode in the tube.
By D-Kuru, CC BY-SA 3.0 at, https://commons.wikimedia.org/w/index.php?curid=4008275
These particles were later identified to be subatomic particles by J. J. Thomson, and named electrons. Thomson was awarded the 1906 Nobel prize for physics for this work. Philipp Lenard also contributed greatly to cathode ray theory, and was awarded the Nobel prize for physics in 1905 for his research on cathode rays and their properties.
Vacuum tubes and modern electronics. The gas ionization method of producing cathode rays (cold cathode) used in early cathode ray tubes were unreliable and required high voltage. It depended on the pressure of the residual air in the tube. When the air was absorbed by the walls of the tube, it would stop working.
A more reliable and controllable method of producing cathode rays was investigated by Hittorf and Goldstein, and rediscovered by Thomas Edison in 1880. In this case, the cathode was made of a wire filament heated red hot by passing a seperate current through it. The high temperature releases electrons from the cathode into the tube through a process called thermionic emission. The first true electronic vacuum tubes, invented in 1904, used this hot cathode technique, and they superseded Crookes tubes. These tubes didn’t need gas in them to work, so they were evacuated to a even lower pressure of around 10−9 atm (10−4 Pa).
In 1906, Lee De Forest found that a small voltage on a grid of metal wires could control a much larger current in a beam of cathode rays passing through a vacuum tube. His invention, called the triode, was the first device that could amplify electric signals, and started the field of electronics. Vacuum tubes made radio and television broadcasting possible, as well as radar, long distance telephone service etc., and were the foundation of consumer electronics until the 1960s when the semiconductor-based transistor brought the era of vacuum tubes to a close.
The technology of manipulating electron beams pioneered in these early tubes was applied practically in the design of vacuum tubes, particularly in the invention of the cathode ray tube (CRT) by Ferdinand Braun in 1897 which was used in television sets and oscilloscopes. It is today still employed in sophisticated devices such as electron microscopes, electron beam lithography and particle accelerators.
