If you are a techie person and happen to be into camping as well, chances are that you have heard about this device called “FlameStower” which supposedly converts fire (rather temperature difference) into electricity. The project attracted $60,143 (well above its goal of $15,000) support on Kickstarter.com and the product is available for purchase now. The following is a Youtube video reviewing this product. However, before taking out your wallet and getting one for yourself, find out how it works and why this particular product is not that efficient (as shown in couple of other reviews on Youtube).
The principle behind this device is the so-called thermoelectric effect which, as the name suggests, describes the conversion between thermal energy (temperature difference) and electricity. The term “thermoelectric effect” actually encompasses three separately identified phenomena: the Seebeck effect, Peltier effect, and Thomson effect. For most part, we just need to understand Seebeck and Peltier effects, since Thomson effect follows the same physics principle as Peltier effect.
Seebeck effect refers to the phenomenon that a potential difference (voltage) will appear between the two ends of a material (e.g., a metal or semiconductor wire) when they are kept at different temperatures. The potential difference is proportional to the temperature difference and the material’s property defined as Seebeck coefficient. The phenomenon was first observed by Estonian-German physicist Thomas Johann Seebeck in 1821, though he originally explained the origin incorrectly.
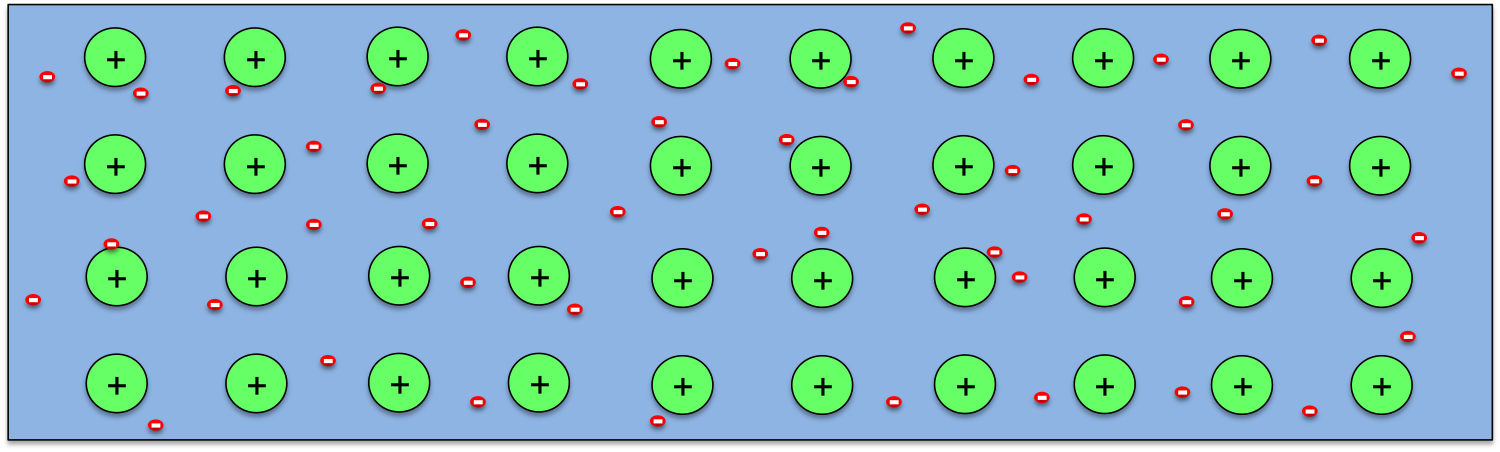
So how can a temperature difference generates potential difference in a wire? As we know, all materials are made of atoms, and atoms contain positively charged nucleus with negatively charged electrons moving around them. The electrons in an atom form layers around the nucleus, with the ones closer to the nucleus being bound more strongly, while the outer ones loosely. So when atoms come together to form a solid, the outer electrons from every atom may break away completely (as in metals) or partially (as in semiconductors). The remaining of the atoms become positively charged ions. This is schematically shown above for the case of metals.
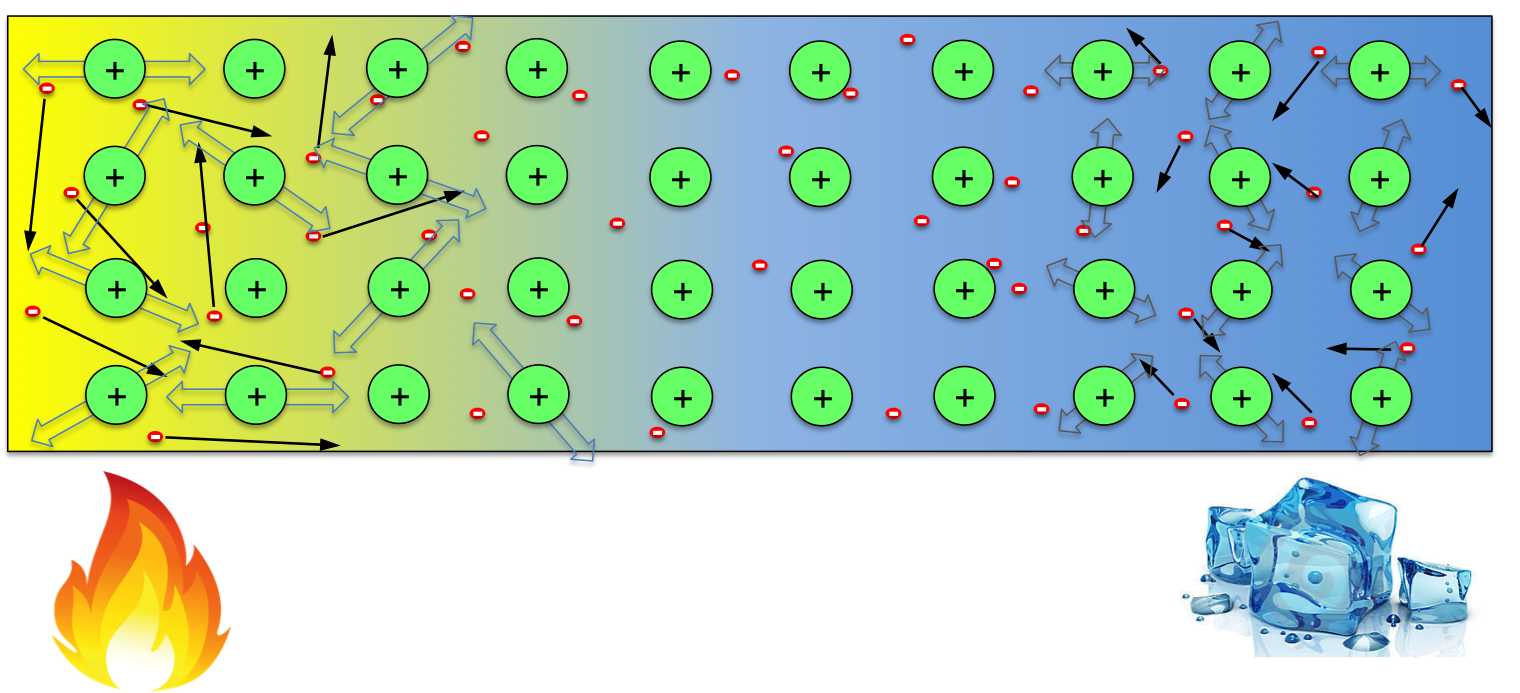
When the temperature across the wire is uniform, the distribution of negative electrons is uniform, which neutralise the positive ions everywhere in the material. However, when one end of the wire is kept at high temperature while the other end kept cool, electrons at the hot end gain more energy, resulting in higher average speed than those at the cool end (indicated by the longer arrows in the following figure). So, within any given time, this would result in more electrons moving to the cool end then those moving back. One would then expect that the hot end of the wire becomes positively charged (some positive ions not compensated). However, note that not only electrons gain energy, but the ions also do. Though they can not move freely, the ions with more energy at the hot end would vibrate more violently, causing more scattering to the electrons when they try to move to the cool end. So the temperate effect on ions favours electrons moving from the cool end to hot end. These two factors compete with each other in every material, whichever wins determines the sign of Seebeck coefficient in it. So in some materials, the hot end becomes positive (high potential), while in others the opposite occurs.
Now let’s look back at the “FlameStower” charger, just beneath the water container should be the thermoelectric device, which sits on the plate that extends out to the heat source. The main issue is that water boils quickly and the temperature difference is hard to maintain, thus its charging capability quickly drops. Note that some of the reviews on Youtube regarding this device mistakenly suggest that heating the water charges the phone, that is absolutely wrong. You want to keep the water as cool for as long as possible, ideally, keep it following and supply cold water constantly.
For more information on thermoelectric effect, please click here.
