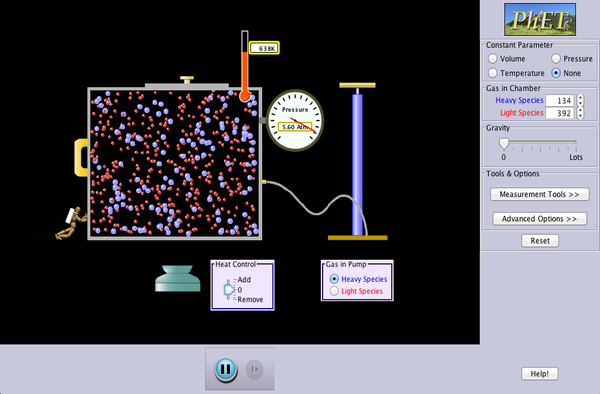
Here is a simulation that helps you to visualise the nature of “temperature”, and what it really means when an object is hot (high temperature) or cold (low temperature).
How to use the simulation?
1, Use the pump to inject some gas molecules into the chamber, not too much, so you can see the movement of molecules. Note that the thermometer indicates that the system starts at 300 K.
2, Heat or cool the chamber by using the heat control at the bottom, give the system a bit time to settle and you will notice that as the movement of the gas molecules becomes more or less violent, the temperature increases or drops accordingly.
3, There are also other parameters to play with. For example, the little guy on the left can push the wall inward, which causes the temperature to rise. (work being done to the gas molecules, raising their kinetic energy).
Conclusions:
1, Everything is made of atoms or ions (when atoms gain or loose electrons, they become ions), and they are in constant motion (they vibrate in solids, move around in liquid with a bit more freedom, and fly around freely in gas).
2, Temperature is a measure of the average kinetic energy of particles that make up the object. An increase in temperature simply means that the particles have gained energy from a source that increases their average kinetic energy.
3, Any temperature triggered occurrence originates from the change in average kinetic energy of particles, e.g. melting of a solid.