Robert Andrews Millikan (March 22, 1868 – December 19, 1953) was an American experimental physicist honored with the Nobel Prize for Physics in 1923 for the measurement of the elementary electronic charge and for his work on the photoelectric effect.

(1868-1953). American physicist.
In 1897, J. J. Thomson had discovered that the cathode ray are made of sub-atomical, negatively charged particles (which he called “corpuscles”, but was later renamed “electrons”), and measured the charge-to-mass ratio of the electron. However, the actual charge and mass values were unknown. Therefore, if one of these two values were to be measured, the other could easily be calculated. In 1909, Millikan conducted a series of experiments to determine the charge carried by a single electron. He began by measuring the course of charged water droplets in an electric field. The results suggested that the charge on the droplets is a multiple of the elementary electric charge, but the measurement was not accurate enough to be convincing. In 1910, he obtained more precise results with his famous oil-drop experiment in which water (which tends to evaporate too quickly) were replaced with oil.
The Milikan Oil-drop Experiments: This experiment involved observing tiny negatively charged oil droplets between a pair of horizontal metal plates (electrodes). The original apparatus used is shown below.

Figure 1, The original apparatus used by Milikan for the oil-drop experiment.
By Unknown – http://chem.ch.huji.ac.il/~eugeniik /history/millikan.html (taken in 2006, now don’t work),
Public Domain, https://commons.wikimedia.org/w/index.php?curid=685647
First, for a single oil droplet, with zero applied electric field, its terminal velocity was measured. At terminal velocity, the drag force equals the gravitational force, and these depend on the droplet’s radius in different ways, so that the radius of the droplet, and therefore its mass and gravitational force, could be determined with the oil’s known density. Second, an adjustable potential difference (V) was applied between the plates to induce an electric field, and the V was adjusted until the drops were suspended in mechanical equilibrium (electrical force = gravitational force). Now using the known electric field, the oil droplet’s charge could be determined.
Detailed workings:
- The electron’s charge was deduced by Millikan based on his “oil drop” experiment. Figure 2 illustrates the schematic drawing of his experimental set up:
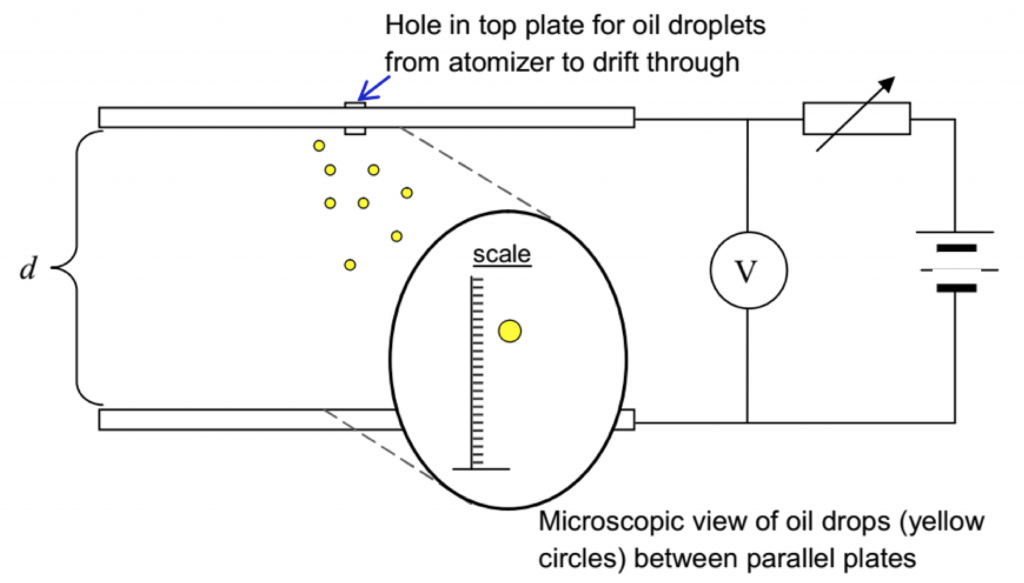
Figure 2, Millikan’s oil drop experimental set up consists of a parallel-plate capacitor with plates separated by a distance d. The mist of oil droplets sprayed out from the atomizer is electrostatically charged and each oil droplet gains a negative charge of –q.
- In the absence of an electric field, as the oil droplets drift through the hole in the top plate, the force acting on each oil droplet is its own weight and air resistance due to the viscosity of the air. The oil droplets are viewed using a microscope. Millikan measured the mass of an oil droplet by observing its drift velocity as it falls.
The frictional force acting on the oil droplet is:![]() where the constant k can be determined by Stoke’s Law and k∝r, radius of the oil droplet and v is the terminal velocity of the oil droplet. As the focus of this article is on Modern Physics, we shall not go in depth with the mathematics of Stoke’s Law. Instead, suffice to know that having obtained the radius of the oil droplet and knowing the density, ρ, of the oil used, it is possible to calculate the mass of the oil droplet:
where the constant k can be determined by Stoke’s Law and k∝r, radius of the oil droplet and v is the terminal velocity of the oil droplet. As the focus of this article is on Modern Physics, we shall not go in depth with the mathematics of Stoke’s Law. Instead, suffice to know that having obtained the radius of the oil droplet and knowing the density, ρ, of the oil used, it is possible to calculate the mass of the oil droplet:
![]()
At terminal velocity, taking upward direction as positive, the total force F on an oil droplet is: F = 0.
- To suspend a particular oil droplet, the uniform electric field between the plates is varied by varying the potential difference between the plates using the rheostat in the circuit until the downward electric force on the oil droplet is equal to its weight.*
The top plate is kept at a positive potential so that the electric field is acting downwards.
Let the charge of the oil droplet under observation be Q = –q.
Taking upward direction to be positive,
Force on oil droplet due to electric field is FE=-q(-E)=qE
Weight of oil droplet, W=-mg (weight is acting downwards.)
Resultant force on oil droplet: F=FE+W=qE-mg=0
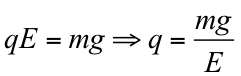
Hence,
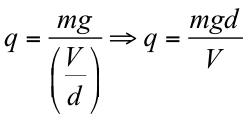
* Notes: The oil drop, carrying negative charges, experiences an upward electric force.
Conclusion:
From the above equation, it is possible to calculate the amount of charge on a particular oil droplet. By repeating the experiment for many droplets, Milikan and his pupil Fletcher confirmed that the charges were all small integer multiples of a certain base value, which was found to be 1.592 X 10−19 C, about 0.6% difference from the currently accepted value of 1.602 X 10−19 C. They proposed that 1.592 X10−19 C was the (negative of the) charge of a single electron.
A bit of History: The oil drop experiment was performed by Robert A. Millikan and his pupil Harvey Fletcher in 1909 to measure the elementary electric charge (an electron’s charge). There are two controversies surrounding this experiment:
- Documents found after Fletcher’s death described how Millikan coerced Fletcher into relinquishing authorship of the discovery as a condition for receiving his PhD.
- Historian Gerald Holton pointed out in 1978 that Millikan recorded more measurements in his journal than he included in his final results, indicating that Milikan selectively omitted a portion of his experimental results in order to present to the scientific community a result with a smaller % error (of 0.6%). If all the results were included, the error would have been 2%. However, according to investigations conducted by David Goodstein, several reasons were provided by Milikan in his more detailed notebooks to account for the failure to generate a complete observation. These include annotations regarding the apparatus setup, oil drop production, and atmospheric effects which invalidated a measurement.
Sources:
[1] Oil Drop Experiment: https://en.wikipedia.org/wiki/Oil_drop_experiment
