Nowadays, electronic gadgets pretty much run our lives. We spend hours browsing the internet, playing games, and chatting with friends using our laptops, smart phones or iPads. Most of us have noticed that the gadgets get hot after a while, sometimes leading to serious consequences. On Youtube, you can find dozens of videos showing people trying crazy tricks with the heat like the one shown below.
If you become curious and try to figure out why electronic gadgets always become hot when running, but never get cold, the almighty Google will tell you that it’s likely because the venting ports are clogged by dust, or the cooling fun is not working properly etc. Unfortunately, that doesn’t really answer the question.
Why do electronic devices become hot when running to begin with? Here is a brief explanation. First of all, temperature is a measure of the average kinetic energy of all particles that make up the material. Particles in a hot object have higher average kinetic energy than those in a cold object.
On a chilling winter day, we feel cold when step outside the house simply because molecules that make up our skin loose kinetic energy to air when they come into contact. On the other hand, our skin molecules gain energy when come into contact with hot summer air, giving us the sensation of hot.
 The materials that make up our electronic gadgets are metals and semiconductors, in which some of the electrons break away from the atoms. When we turn the device on, the electromotive force provided by the battery drives the electrons to move, taking information from one component to another and producing the images and sounds.
The materials that make up our electronic gadgets are metals and semiconductors, in which some of the electrons break away from the atoms. When we turn the device on, the electromotive force provided by the battery drives the electrons to move, taking information from one component to another and producing the images and sounds. 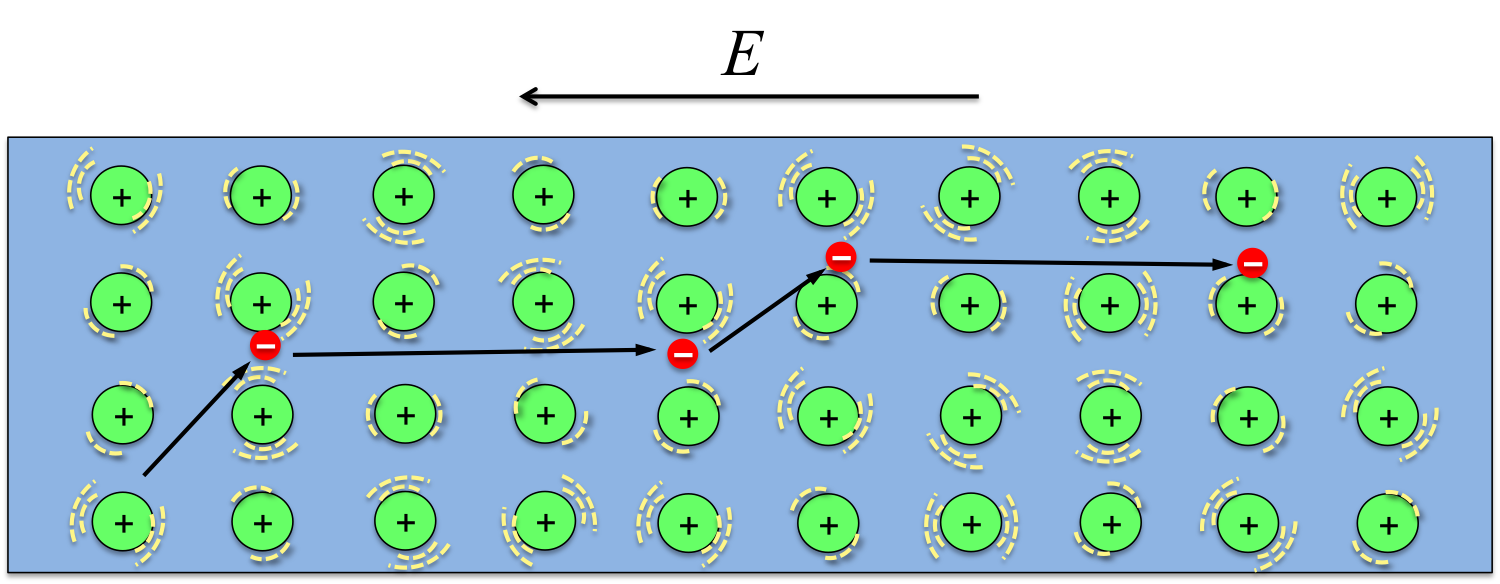
When electrons move in the metals or semiconductors of our electronic devices, collisions with the ions that make up the solids are inevitable. The average distance an electron can travel between two consecutive collisions is called the mean free path, during which electrons accelerate and gain more energy. During the collision, electrons will release the energy it gains from the electric (E) field to the positive ions and make them vibrate more violently, thus the solid becomes hotter (more kinetic energy of the ions means higher temperature).
In fact, such collisions is the reason for materials’ electrical resistance. The more collisions that occur, the higher the resistivity of the material. If we want to eliminate the heating problem all together, we will need a material in which electrons run completely free without colliding into any of the ions. Indeed, there are such materials, and they are called superconductors! Unfortunately, the superconducting property only exists at very low temperatures at the moment.
